電催化科研社 2024年10月06日 07:55 上海
研究背景
水分解產(chǎn)生氫氣作為一種可持續(xù)的氫能源生產(chǎn)技術(shù),已經(jīng)引起了廣泛的關(guān)注。然而,這一過程中的氧析出反應(yīng)(OER)是限制其效率的關(guān)鍵因素,因為它涉及高能中間體的四個電子和四個質(zhì)子的協(xié)同轉(zhuǎn)移。目前的研究致力于開發(fā)高性能的OER催化劑,以提高反應(yīng)效率和穩(wěn)定性。
工作內(nèi)容
本研究通過同時激活金屬和晶格氧位點,構(gòu)建了一個兼容的多機制催化劑R-NiFeOOH@SO4。該催化劑在堿性介質(zhì)中表現(xiàn)出卓越的OER活性,能夠驅(qū)動高達100/500mA cm-2的電流密度,并展現(xiàn)出超過300小時的穩(wěn)定性。研究通過一系列的實驗和表征手段,包括電化學(xué)測試、原位拉曼光譜、原位衰減全反射傅里葉變換紅外光譜以及密度泛函理論計算,深入探討了催化劑的結(jié)構(gòu)和性能之間的關(guān)系。特別是,通過引入Fe和S作為調(diào)制劑,優(yōu)化了Ni位點的電子結(jié)構(gòu),進而促進了OER過程中金屬和晶格氧位點的協(xié)同氧化還原。
工作創(chuàng)新點
1.多機制兼容催化劑的設(shè)計:本研究成功地設(shè)計了一種能夠同時利用金屬和晶格氧位點進行OER的兼容多機制催化劑。
2.電子結(jié)構(gòu)的優(yōu)化:通過引入Fe和S作為調(diào)制劑,優(yōu)化了NiMoO4?xH2O的電子結(jié)構(gòu),從而提高了OER活性。
3.長時間穩(wěn)定性:R-NiFeOOH@SO4催化劑在堿性介質(zhì)中表現(xiàn)出超過300小時的穩(wěn)定性,這對于實際應(yīng)用具有重要意義。
4.深入的機理研究:通過原位光譜和DFT計算,揭示了催化劑在OER過程中的結(jié)構(gòu)變化和活性位點的動態(tài)行為。

Fig. 1 Preparation and structures of catalysts. a Synthetic route of nanocoral-like NiMoO4.xH2O@Fe,S, with the inset zoomed-in images presenting the surface structure evolution of the nanorods. FESEM, TEM, and High-resolution TEM images of b, f NiMoO4.xH2O, c, g NiMoO4.xH2O@Fe,S and d, e, h R-NiFeOOH@SO4. (The inset shows a magnified view, and the inset in Fig1. g is the corresponding selected area electron diffraction pattern). i HAADF-STEM image and corresponding EDS elemental mappings of Ni, Mo, O, Fe, S for R-NiFeOOH@SO4.
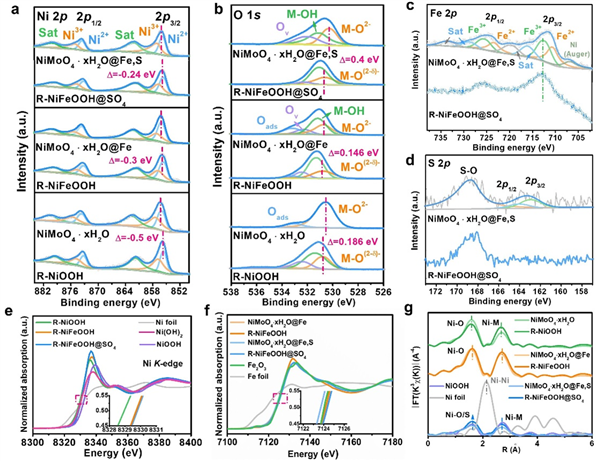
Fig. 2 Electronic and coordination structures of catalysts. High-resolution XPS of a Ni 2p, b O 1s, c Fe 2p, and d S 2p for obtained samples before and after CV activation. e Normalized Ni K-edge, f Fe K-edge XANES spectra of obtained samples and selected reference materials. The inset shows a magnified view of the selected area, x-axis: Energy (eV), y-axis: Normalized absorption (a.u.). g Fourier transformed k3χ(R) Ni K-edge spectra of obtained samples before and after CV activation.
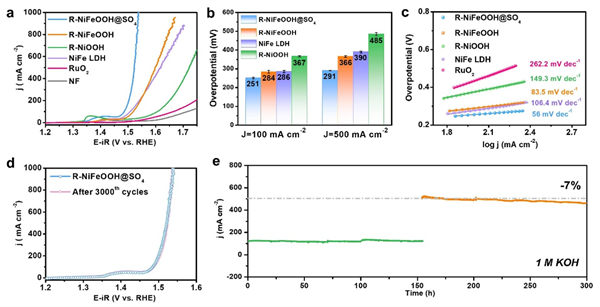
Fig. 3 Electrocatalytic OER performance of catalysts. a OER Polarization curves with 80% iR compensation (The electrode working area is 0.5 cm × 0.5 cm, and the solution resistance used for compensation is approximately 2.3±0.2 Ω), and b corresponding overpotentials of catalysts at 100 and 500 mA cm-2 (The standard deviations were obtained through three reduplicative measurements in Supplementary Fig. 31). c Tafel plots of catalysts. d LSV curves before and after 3000 CV cycles, and e Chronoamperometry curves without iR compensation of R-NiFeOOH@SO4 in 1 M KOH at 0.65 and 0.85 V vs. Hg/HgO.
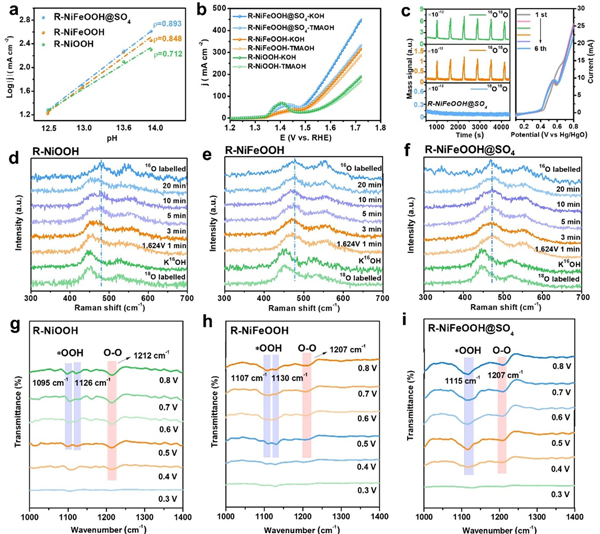
Fig. 4 Compatible with AEM-LOM OER catalysis analysis. a The relationship between the logarithm of current density of catalysts at the potential of 1.7 V versus RHE and pH. b OER Polarization curves of catalysts in 1 M KOH and 1 M TMAOH, respectively, without iR compensation. c Detected DEMS signals without any correction or subtraction of 16O16O, 16O18O and 18O18O for R-NiFeOOH@SO4 relative to time and corresponding LSV curves, no iR compensation. In-situ Raman spectra of 18O-labelled d R-NiOOH, e R-NiFeOOH and f R-NiFeOOH@SO4 measured at 1.624 V vs RHE in 0.1 M KOH with H216O. The Raman spectra of the 16O labeled sample is placed on the top for comparative analysis. In situ ATR-IR spectra of g R-NiOOH, h R-NiFeOOH, i R-NiFeOOH@SO4.
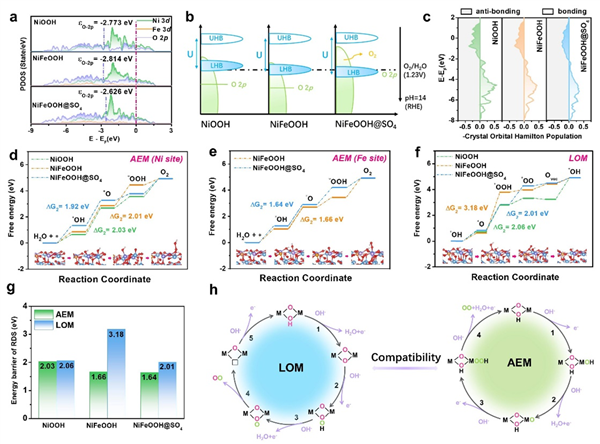
Fig. 5 DFT calculation analysis. a Projected density of states, b Schematic band diagrams (UHB: upper Hubbard band, LHB: lower Hubbard band) and c COHP of the Ni-O bond. Gibbs free energy diagrams of the AEM pathway on d Ni sites and e Fe sites, and f LOM pathway. g The energy barrier of RDS for AEM and LOM. Above data are based on NiOOH, NiFeOOH and NiFeOOH-SO4. h Schematic illustration of the AEM and LOM pathway on NiFeOOH@SO4.
原文信息
Luo, X., Zhao, H., Tan, X. et al. Fe-S dually modulated adsorbate evolution and lattice oxygen compatible mechanism for water oxidation. Nat Commun 15, 8293 (2024).
https://doi.org/10.1038/s41467-024-52682-y